ARBs in HFrEF (ELITE I & II, CHARM-Alternative, CHARM-Added & Val-HeFT)
Bottom-line: In patients with HFrEF with suboptimal beta-blocker use, ARBs reduce the risk of death or HF hospitalization (NNT 40-80/year; mainly from reduced HF hospitalizations), whether added to ACEI or used instead in ACEI-intolerant individuals. ARBs increase the risk of discontinuation due to adverse events when added to ACEI (NNH ~20-40), primarily from hypotension, renal dysfunction & hyperkalemia.
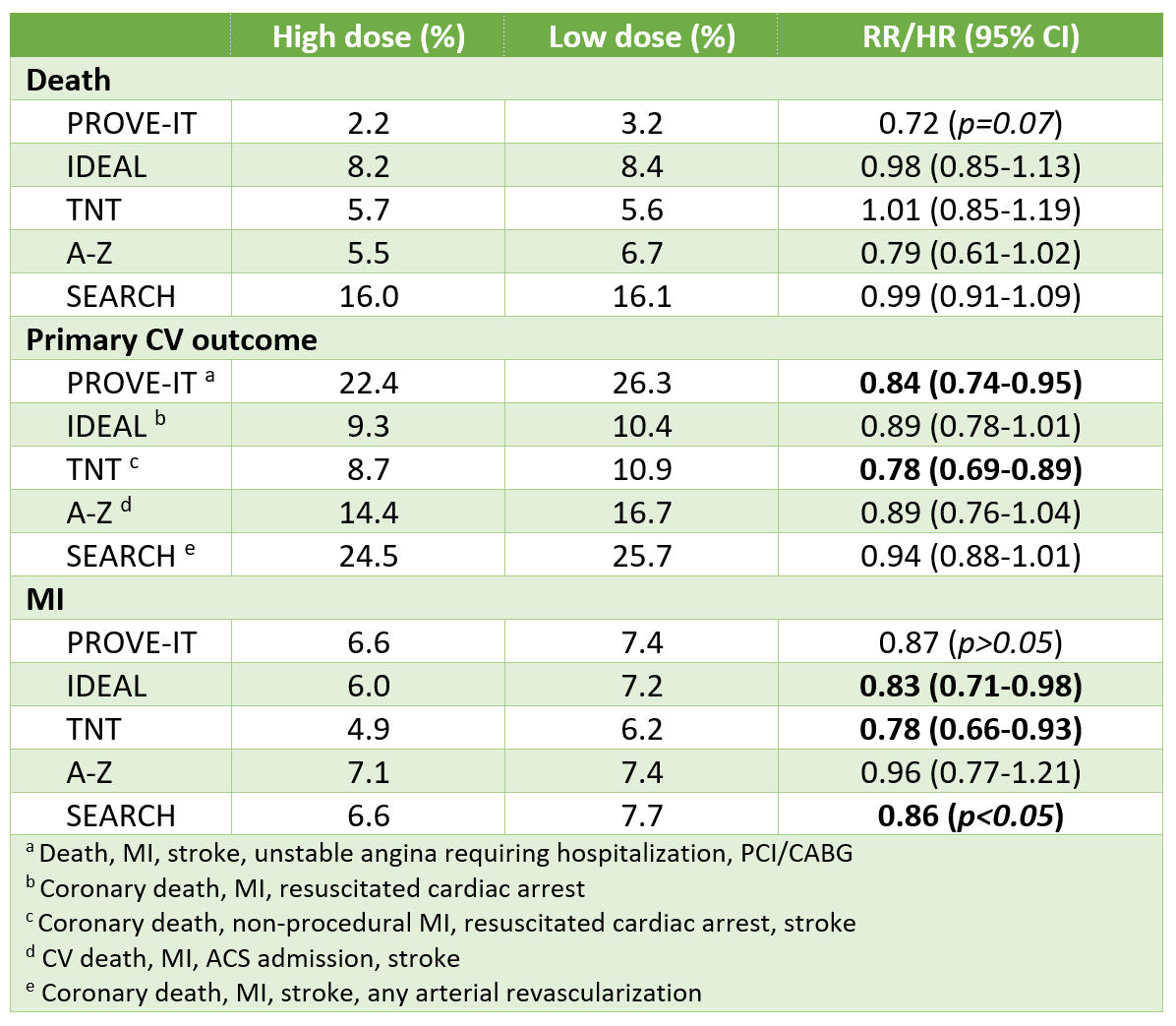
Results
Safety
- CHARM-Alternative
- Any adverse event or lab abnormality: Candesartan 21.5% vs placebo 19.3% (p=0.23)
- Hypotension leading to D/C: 3.7% vs 0.9% (NNH 36)
- SCr increase leading to D/C: 6.1% vs 2.7% (NNH 30)
- Hyperkalemia: 1.9% vs 0.3% (NNH 63)
- Any adverse event or lab abnormality: Candesartan 21.5% vs placebo 19.3% (p=0.23)
- CHARM-Added
- Any adverse event or lab abnormality: Candesartan 24.2% vs placebo 18.3% (NNH 17)
- Hypotension leading to D/C: 4.5% vs 3.1%
- SCr increase leading to D/C: 7.8% vs 4.1%
- Hyperkalemia: 3.4% vs 0.7%
- Any adverse event or lab abnormality: Candesartan 24.2% vs placebo 18.3% (NNH 17)
- Val-HeFT
- Drug D/C: Valsartan 9.9% vs placebo 7.2% (NNH 37)
- Dizziness 1.6% vs 0.4%
- Hypotension: 1.3% vs 0.8%
- Renal impairment: 1.1% vs 0.2%
- Drug D/C: Valsartan 9.9% vs placebo 7.2% (NNH 37)
Generalizability of trials adding ARB to ACEI
- CHARM-Alternative specifically enrolled patients intolerant of ACEI & therefore not receiving these agents, whereas 93% of patients enrolled in Val-HeFT received ACEI therapy
- Results of CHARM-Added were similar to those of Val-HeFT (HR 0.85 for primary outcome & 0.83 for HF hospital admission)
- Patients in both trials had overall poor optimization of other HF meds (35-55% on beta-blockers, 5-25% on mineralocorticoid receptor antagonist) at baseline.
Internal validity
Low risk of allocation, performance, detection and attrition bias, as all trials were randomized, allocation concealed, double-blind trials with blinded outcome adjudication, loss-to-follow-up <1% and intention-to-treat analysis.