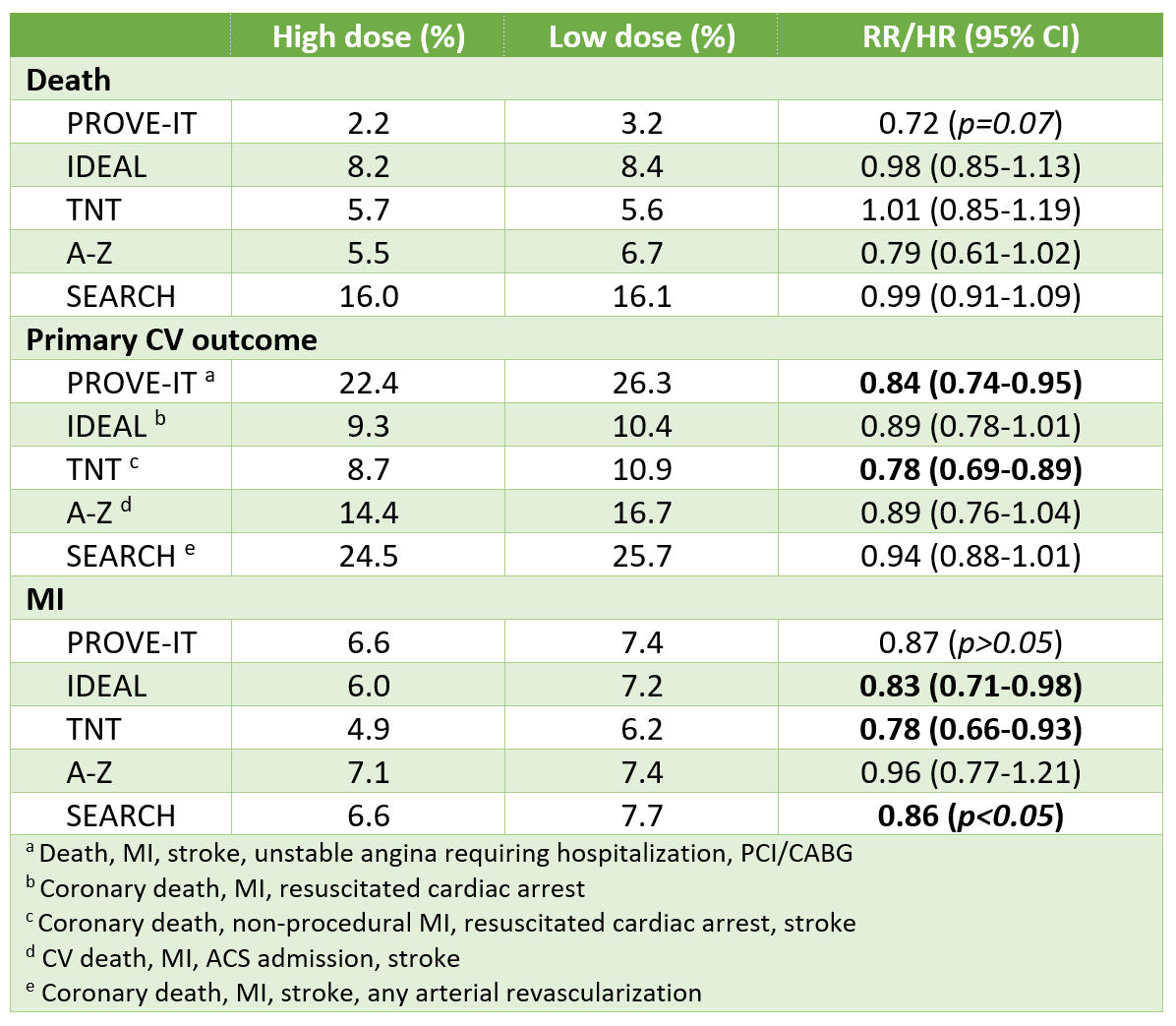
Silverman MG, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: A systematic review and meta-analysis. JAMA 2016;316:1289-97.
Clinical question: Does reduction of CV outcomes with lipid-lowering therapy correlate with degree of LDL-lowering?
Bottom line:
The value of this study is mechanistic; it does not provide clinical guidance.
The analysis demonstrates that CV outcome reduction of proven lipid-lowering drugs is closely associated with degree of LDL-lowering. This supports the lipid hypothesis, i.e. that lipid-lowering drugs which lower CV risk outcomes (statins, bile acid sequestrants, & ezetimibe) do so primarily by lowering LDL.
This study does not validate a particular lipid target, nor does it support using interventions with neutral, harmful or conflicting evidence (fibrates, niacin, or CETP inhibitors) to achieve a lipid target.
Search
- Databases: MEDLINE, Embase
- Timeframe: 1966 to July 2016
- Inclusion criteria:
- Randomized controlled trials (RCTs)
- Compared (1) LDL-lowering intervention to control/placebo or (2) more vs less intensive statin therapy
- Reported cardiovascular outcomes including MI
- Duration of at least 6 months
- At least 50 events
- Exclusion criteria: Trial with populations with "significant competing risks", including heart failure & chronic kidney disease
- Additional measures for comprehensiveness:
- References lists of identified studies, review and meta-analyses
- Reviewed abstracts of major cardiovascular meetings held in past 2 years (no mention of which)
- Contacted content experts
Results of systematic review
- Included 49 RCTs (n=312,175)
- Statin (25 trials)
- Fibrate (9 trials)
- Diet (4 trials)
- CETP inhibitor (3 trials)
- Niacin (3 trials)
- Bile acid sequestrants (2 trials)
- PCSK9 inhibitor (2 trials)
- Ezetimibe (1 trial)
- Ileal bypass surgery (1 trial)
Results of the meta-analysis
- Mean follow-up 4.3 years
- Mean ~absolute reduction in LDL vs placebo in trials
- PCSK9 inhibitor -1.85 mmol/L
- Ileal bypass -1.6 mmol/L
- Bile acid sequestrant -0.90 mmol/L
- Statin -0.85 mmol/L (all drugs & doses pooled)
- Diet -0.75 mmol/L
- Niacin -0.35 mmol/L
- Ezetimibe -0.3 mmol/L
- Fibrate -0.25 mmol/L
- ~23% relative risk reduction (RRR) in major vascular events per 1 mmol/L reduction in LDL with any interventions except CETP inhibitors
Considerations & limitations
- Generalizability & internal validity
- Investigators excluded studies that included patients with "significant competing risk", which includes some of the landmark "negative" statin trials (CORONA, GISSI-HF, 4D, etc)
- Results of this analysis don't apply to the subpopulations of these studies (primarily heart failure & chronic kidney disease)
- The analysis may overestimate the true relative risk reduction since this exclusion criterion primarily excluded "negative" studies.
- Numerous differences in populations between trials, including
- Era (e.g. 1st fibrate/niacin trial conducted in 60s, most statin trials conducted in 1990s-2000s)
- Primary vs secondary prevention
- LDL before initiation of study treatment & background CV therapies
- Results
- Although interventions produced comparable RRRs in CV outcomes per 1-mmol/L reduction in LDL, actual achievable LDL reduction & therefore realistic CV reductions with each agent are quite different
- This study evaluated a composite CV outcome, which bundles together outcomes of different severity & importance to patients. Different interventions may have the same effect on a composite CV outcome, but not specific components. For example, statins reduce every type of CV event (death, MI, stroke, revascularization), whereas fibrates only reduce non-fatal MI, but not death or stroke.