TOPCAT & FINEARTS-HF: Spironolactone & finerenone in heart failure with ejection fraction >40%
TOPCAT (spironolactone) & FINEARTS-HF (finerenone)
Bottom line:
In patients with HF with EF >40% (i.e., HF with mildly-reduced or preserved EF), MRAs reduced the risk of HF hospitalization vs placebo, did not reduce the risk of dying, and increased the risk of hypotension, hyperkalemia, and eGFR reduction.
For every 1000 patients treated with an MRA per year, 15 HF hospitalizations would be avoided, but there would be 20-30 more people with hypotension (with or without symptoms), 70-130 more patients with eGFR reduced by >30%, and 70-80 more patients with K >5.5 mmol/L.
There is no direct head-to-head comparison of finerenone vs spironolactone. Efficacy & safety seem to be similar with these 2 drugs; this likely represents a class effect of MRAs in HFmrEF/HFpEF.
TOPCAT
TOPCAT: Spironolactone for heart failure with preserved ejection fraction. NEJM 2014;370:1383-92
TOPCAT-Americas: Circulation 2015;131:34-42
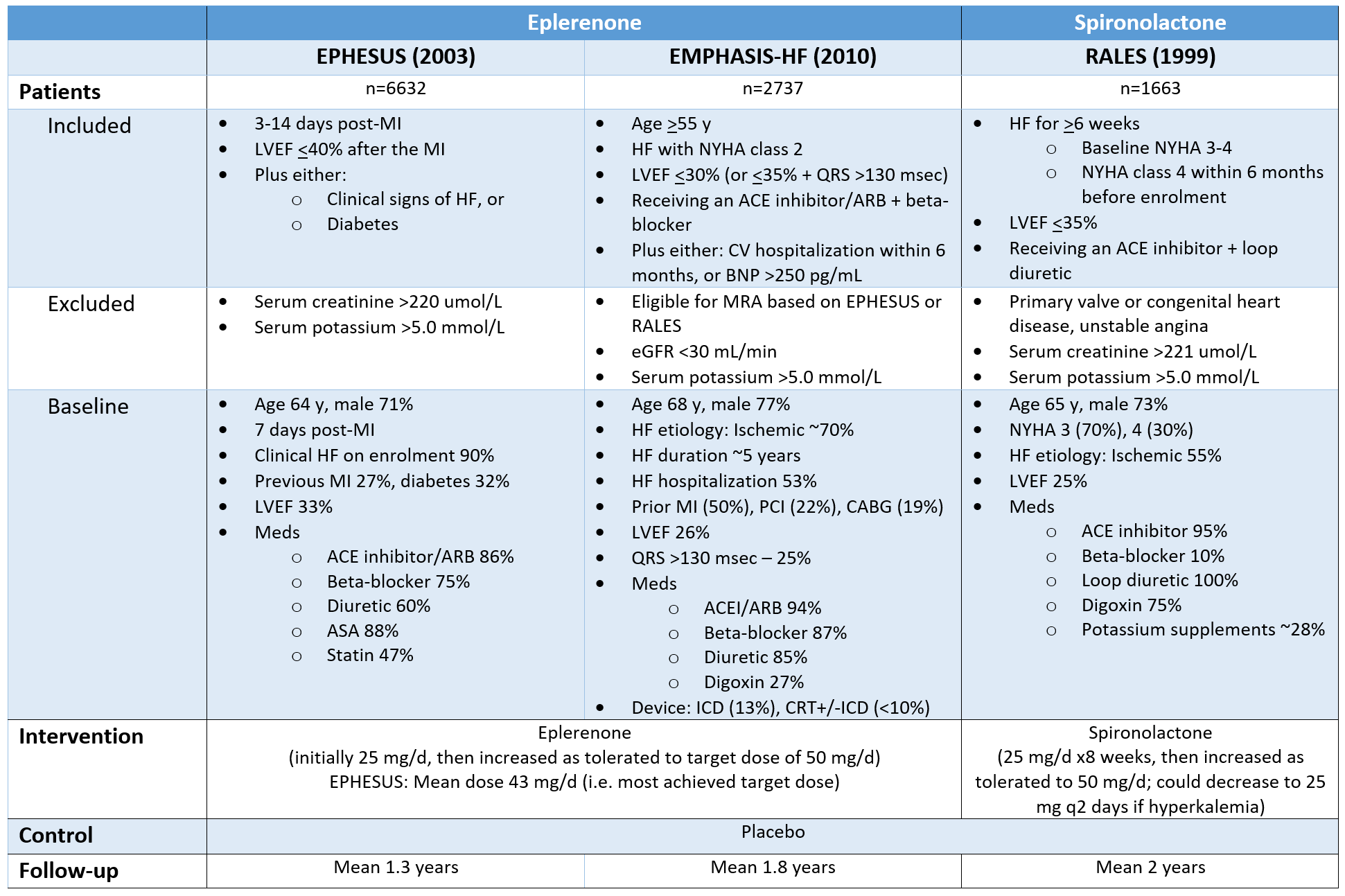
Patients (n=3445)
Inclusion
Age 50+
Clinical HF
LVEF >45% (measured within 6 months prior to randomization & after any prior ACS)
Controlled SBP (<140, or <160 if receiving 3+ antihypertensives)
Either,
1+ HF-related hospitalization in prior 12 months, or
BNP >100 pg/mL (or N-terminal pro-BNP >360 pg/mL) within 30 days, not explained by another disease entity
Exclusion
Pericardial constriction
Hemodynamically significant uncorrected primary valvular heart disease
Infiltrative or hypertrophic obstructive cardiomyopathy (HoCM)
Stroke, MI or CABG in past 90 days
AF with resting HR >90 bpm
Heart transplant recipient or currently implanted LVAD
Chronic pulmonary disease: Requiring home O2 or PO steroids, hospitalization for exacerbation within 12 months, or significant in the opinion of the investigator
"Average" patient
Age 69 y (age 75+ 27%), female 52%, white 89%
HF characteristics
Hospitalization in last 12 months 71%
NYHA functional class 1 (3%), 2 (64%), 3 (33%), 4 (<1%)
BNP 236
LVEF 56%
BP 130/80 mm Hg
Meds: Diuretic 80%, ACEI/ARB 85%, beta-blocker 75%
Intervention: Spironolactone
Initial dose: 15 mg PO daily x4 weeks
If initial dose tolerated, increased to 30 mg daily
If HF still symptomatic at month 4, increased to 45 mg daily
Monitoring: Measurement of SCr & serum K required <1 week after start or change of study drug dose
Comparator: Matching placebo
Outcomes @ mean 3.3 y
Efficacy
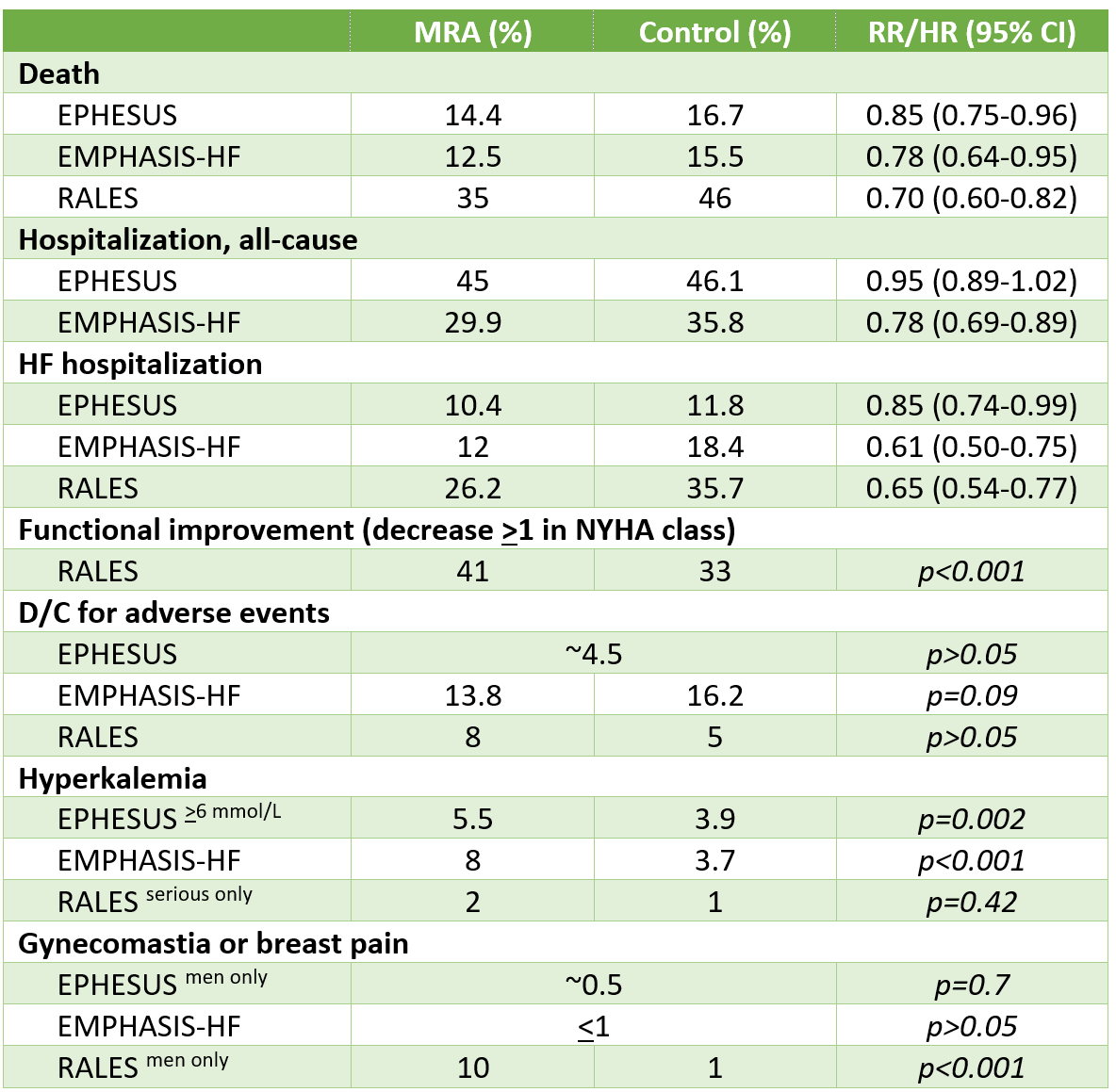
Death: Spironolactone 14.6% versus placebo 15.9%, hazard ratio (HR) 0.91 (95% confidence interval 0.77-1.08)
Hospitalization for any cause: 44.5% vs 46%, HR 0.94 (0.85-1.04)
Primary outcome (CV death, aborted cardiac arrest, HF hospitalization): 18.6% vs 20.4%, HR 0.89 (0.77-1.04)
Kansas City Cardiomyopathy Questionnaire (KCCQ; 100-point scale, minimal clinically important difference -5): Versus placebo, +1.5 at 4 months & +1.9 at 36 months (p=0.02)
Safety
Discontinuation of study drug: 34.3% vs 31.4%
Doubling of SCr: 10.2% vs 7% (NNH 32)
Hyperkalemia (serum K >5.5 mmol/L): 18.7% vs 9.1% (NNH 11)
Internal validity
Low risk of bias characteristics: Randomized, allocation-concealed, double-blind trial analyzed using intention-to-treat population; loss-to-follow-up on vital status ~4%
Subgroup analysis: Primary outcome varied based on region (Americas vs Russia/Georgia, p<0.001 for interaction)
Americas: Spironolactone 27.3% vs placebo 31.8%, HR 0.82 (0.69-0.98) (NNT=23)
Russia/Georgia: 9.3% vs 8.4%, HR 1.10 (0.79-1.51)
Credibility of this subgroup effect has been increased by a substudy demonstrating that participants from Russia were far more likely than those from North America to have no detectable serum concentrations of spironolactone metabolites. This indicates that significantly more participants from Russia did not receive the study drug, and raises the potential of misconduct at these study sites. Therefore, the results of the 'Americas' subgroup may be the most accurate reflection of the effect of spironolactone in HFpEF.
Secondary outcomes from the Americas subgroup:
HF hospitalization: 20.8% vs 24.5%, HR 0.82 (0.67-0.99) (NNT=28 over 3.3 years)
Hyperkalemia: 25.2% vs 8.9% (NNH=7)
FINEARTS-HF
Patients (n=6001)
7463 screened -> 6016 randomized -> 6001 analyzed
Inclusion:
Age >=40
HF with NYHA 2-4
Outpatient or hospitalized for heart failure (hemodynamically stable off inotropes)
Receiving diuretic >=30 days
LVEF >=40% measured in last year (could be <40% before)
Structural heart abnormality (LA enlargement, LVH)
NTproBNP >=300 pg/mL in SR or >=900 pg/mL in AF
Key exclusions:
SBP <90 mm Hg
eGFR <25
K >5.0 mmol/L; prior hyperkalemia with MRA
Untreated HTN; alternative causes for HF symptoms
“Average” trial patient:
72 y/o, 46% female, white 79%/Asian 17%
PMHx: HTN 88%, T2DM 42%, AF 38%, prior LVEF <40% 5%
HF characteristics
Prior HF hospitalization 60%
Time since HF event: <=1 week 20%, 7-90 days 34%, >3 months/none 46%
NYHA 2 (69%), 3 (30%), 4 (<1%)
LVEF 52% (36% <50%)
NTproBNP ~1000
SBP 129 mm Hg, K 4.4 mmol/L, eGFR 62
Meds: Loop diuretic 87%, beta-blocker 85%, ACEI/ARB ~70%, ARNI 8-9%, SGLT2i 13-14%, GLP1-RA 2-3%
Intervention: Finerenone
If eGFR >60:
Starting dose: Finerenone 20 mg PO once daily
Increase to 40 mg PO once daily after 4 weeks (target dose)
Dose range 10-40 mg daily
If eGFR 25-60:
Starting dose: Finerenone 10 mg PO once daily
Increase to 20 mg PO once daily after 4 weeks (target dose)
Dose range: 10-20 mg daily
Comparator: Matching placebo
Outcomes @ median 2.7 y
Efficacy
Death: 16.4% vs 17.4% (HR 0.93, 95% CI 0.83-1.06)
Primary outcome (composite of cardiovascular death or worsening HF event [first or recurrent hospitalization or urgent visit for HF]):
Finerenone 14.9 vs placebo 17.7 per 100 patient-years
Rate ratio 0.84, 95% confidence interval (CI) 0.74-0.95
Similar result using traditional “time to first event”: 20.8% vs 24.0% (hazard ratio [HR] 0.84, 95% CI 0.76-0.94)
Results consistent across subgroups
Kidney composite outcome (sustained eGFR decrease ≥50%, sustained eGFR to <15, or initiation of long-term dialysis or kidney transplantation): 2.5% vs 1.8% (HR 1.33, 95% CI 0.94-1.89)
NYHA improvement at 12 months: ~19% in both groups (odds ratio 1.01, 95% CI 0.88-1.15)
Change from baseline in Kansas City Cardiomyopathy Questionnaire (KCCQ) total symptom score: +1.6 (95% CI +0.8 to +2.3) out of 100
Safety
Discontinued study drug: 20% in both groups
SBP <100 mm Hg (symptoms not specified): 18.5% vs 12.4%
Hyperkalemia (K >5.5 mmol/L): 14.3% vs 6.9%
>6: 3% vs 1.4%
“Investigator-reported hyperkalemia”: 9.7% vs 4.2%
Death from hyperkalemia: 0% in both groups
Serum creatinine increase to >221 umol/L: 2% vs 1.2%
Internal validity
Low risk of bias
Allocation bias: Randomized, allocation concealed
Performance & detection bias: Patients, clinicians, outcome assessors unaware of treatment assignment
Attrition bias: Intention to treat analysis; low loss to follow-up (~0.1%)
Meta-analysis
Pooled results of TOPCAT + FINEARTS-HF:
Composite of cardiovascular death or HF hospitalization pool: HR 0.87 (95% CI 0.79-0.95), 1.5% absolute risk reduction
Death: HR 0.94 (0.85-1.03)
Safety: Similar absolute increase (vs placebo) with both spironolactone & finerenone
SBP <90 mm Hg:
TOPCAT: Spironolactone 4% vs placebo 2%
FINEARTS-HF: Finerenone 5% vs placebo 3%
eGFR reduction >30%:
TOPCAT: 31% vs 24%
FINEARTS-HF: 35% vs 22%
K >5.5 mmol/:
TOPCAT: 12% vs 5%
FINEARTS-HF: 15% vs 7%