EMPEROR-Reduced: Empagliflozin in patients with heart failure with reduced ejection fraction with or without type 2 diabetes
Bottom line:
Among patients with symptomatic heart failure with reduced ejection fraction (HFrEF) (with or without type 2 diabetes), empagliflozin reduced the risk of a composite of CV death or HF hospitalization vs placebo (NNT 19) at 1.3 years.
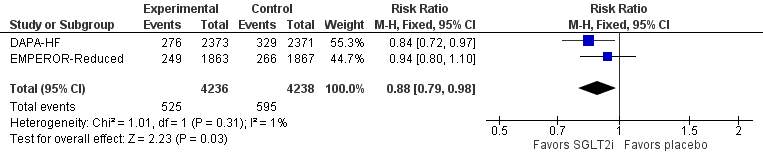
These results are consistent with those of the DAPA-HF trial, and together these trials show that SGLT2 inhibitors reduce death from any cause (NNT 59) over 1.3-1.5 years.
Empagliflozin increased the risk of genital infections (i.e. yeast infections; NNH 91), which are generally self-limiting or resolve with over-the-counter treatment. Empagliflozin did not increase any other adverse effects in this trial.
Patients (n=3730 randomized from 7220 screened)
Inclusion criteria
Heart failure (NYHA 2-4) with reduced ejection fraction (≤40% or less)
Receiving background guideline-directed medical therapy (GDMT) & cardiac device therapy as indicated
Elevated NT-proBNP with threshold dependent on heart rhythm & LVEF
Normal sinus rhythm (AF)
LVEF ≤30%: ≥600 pg/mL (≥1200 pg/mL)
LVEF 31-35%: ≥1000 pg/mL (≥2000 pg/mL)
LVEF 36-40%: ≥2500 pg/mL (≥5000 pg/mL)
Key exclusion criteria
eGFR <20
Symptomatic hypotension or SBP <100 mm Hg
BMI ≥45
Heart transplant recipient, listed for heart transplant, LVAD in situ
Infiltrative or accumulation cardiomyopathy, muscular dystrophy, HoCM, or cardiomyopathy with reversible causes (e.g. Takotsubo, tachyarrhythmia-related)
Baseline
Age 67, 24% female, 70% white & 18% Asian
NYHA 2 (75%), 3 (24%), 4 (0.5%)
Mean LVEF 27% (~73% ≤30%)
Ischemic cardiomyopathy 52%, HF hospitalization in last year 31%, AF 37%, diabetes 50%
HFrEF medications: ACEI/ARB 70%, ARNI 19%, BB 95%, MRA 71% (% achieving target doses not described)
Devices: ICD 31%, CRT 12%
SBP 122, HR 71
Interventions: Empagliflozin vs placebo
Intervention: Empagliflozin 10 mg PO once daily (fixed dose)
Control: Matching placebo
Co-intervention in both groups: Background HFrEF GDMT
Outcomes @ median 16 months
Efficacy
Primary outcome (CV death or HF hospitalization): Empagliflozin 19.4% vs placebo 24.7%
Hazard ratio (HR) 0.75 (95% confidence interval [CI] 0.65-0.86)
NNT 19 over 16 months (or NNT~25/year)
Death from any cause: 13.4% vs 14.2% (HR 0.92, 95% CI 0.77-1.10)
KCCQ-Clinical Summary Score change at 1 year: +5.8 vs +4.1 (difference 1.7, 95% CI +0.5 to +3.0)
KCCQ-CSS is out of 100, minimal clinically important difference ≥5)
HF hospitalization: 13.2% vs 18.3% (HR 0.69, 95% CI 0.59-0.81)
Composite renal outcome (chronic dialysis, renal transplant, sustained eGFR reduction ≥40%)
Mean change in eGFR (mL/min/1.73 m^2)/year: -0.55 vs -2.28, difference 1.73 (1.10-2.37)
Quality of life (Kansas City Cardiomyopathy Questionnaire measured at month 3, 8, 12 & end-of-study):
Safety
Genital infections: 1.7% vs 0.6% (p=.005)
Complicated in 0.3% in both groups
No difference:
Stopped study drug prematurely: Empagliflozin 16,3% vs placebo 18.0%
Symptomatic hypotension: 5.7% vs 5.5%
SBP change: -0.7 mm Hg (-1.8 to +0.4)
Volume depletion: 10.6% vs 9.9%
Ketoacidosis: 0 in both groups
UTI: 4.9% vs 4.5%
Hypoglyemic event: 1.4% vs 1.5%
Lower limb amputation: 0.7% vs 0.5%
Internal validity: Low risk of bias
Allocation concealment by use of an interactive-response system (low risk of allocation bias)
Blinding of participants & clinicians with use of matching placebo (low risk of performance & detection bias)
Use of ITT analysis with 1.1% lost to follow-up (low risk of attrition bias)
Other considerations
Practical tip: Empagliflozin is currently available in 10-mg and 25-mg tablets. The 10 mg/d dose used in EMPEROR-Reduced was based on the lack of difference between 10 mg/d and 25 mg/d in the EMPA-REG trial. The 25-mg tablets can be split in half, and the patient can be instructed to take half a 25-mg tablet (=12.5 mg) once a day. This simple intervention would cut the cost of this therapy by half (e.g. reducing the cost to ~$500/year in Canada).
Results of a meta-analysis (without systematic review) of the 2 large HFrEF SGLT2i trials, EMPEROR-Reduced & DAPA-HF, showed no heterogeneity in efficacy outcomes between these trials. These replicate findings confirm the efficacy of SGLT2 inhibitors in HFrEF, strongly suggest a class effect, and also show no heterogeneity in the effect on death from any cause.
Most exclusions at screening were due to patients not meeting the trial’s fairly strct NT-proBNP criteria (n=3314; 74.6% of those excluded)
However, this does not impact generalizability, as results are consistent with DAPA-HF, which had more lenient NT-proBNP criteria (>900 if AF/atrial flutter, >400 if HF hospitalization within 1 year, otherwise >600)