EMPEROR-Preserved: Empagliflozin in heart failure with preserved or mildly-reduced ejection fraction
References:
EMPEROR-Preserved secondary paper on worsening heart failure events. Circulation 2021
EMPEROR-Preserved secondary paper on quality of life. Circulation 2021
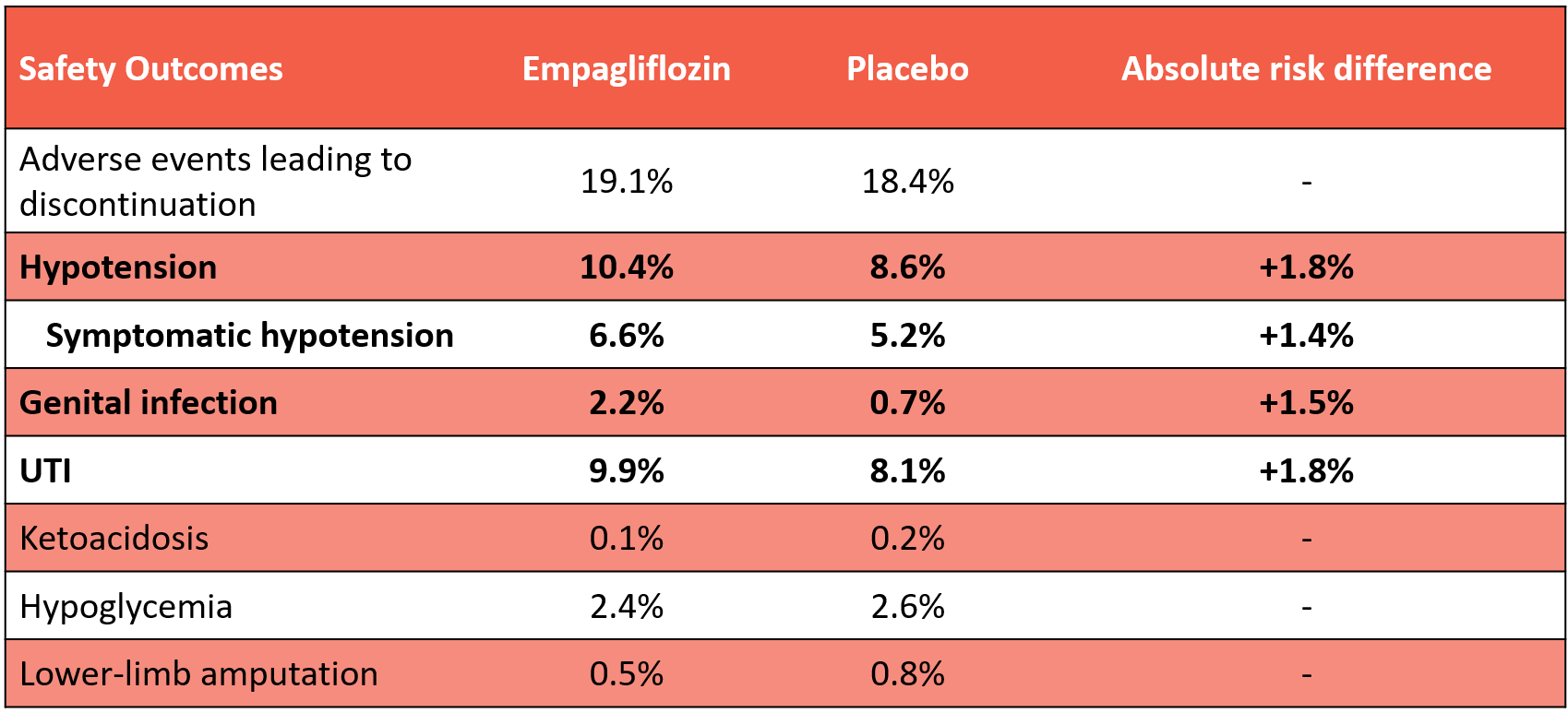
Bottom Line: In patients with symptomatic heart failure with preserved (≥50%; HFpEF) or mildly-reduced (41-49%; HFmrEF) ejection fraction, empagliflozin reduced the risk of HF hospitalization vs placebo (-3.2%) and increased the probability of a clinically important improvement in quality of life (+3.8%), but did not reduce deaths or total hospitalizations at 2.2 years. Empagliflozin increased the risk of symptomatic hypotension (+1.4%), genital fungal infections (+1.5), and UTIs (+1.8%).
Patients (n=5988)
11,583 screened -> 5988 randomized
Most common reasons for exclusion:
78% NT-proBNP below inclusion criteria threshold
5% LVEF <40%
4% exclusion criteria based on safety
Enrolment 2017-April 2020
23 countries
Included: Symptomatic chronic HFpEF or HFmrEF with elevated natriuretic peptide
Chronic HF (≥3 months)
NYHA 2-4
LVEF >40% without any prior LVEF ≤40%
NT-proBNP
>300 pg/mL if in sinus rhythm
>900 pg/mL if in atrial fibrillation
Either
Structural heart disease (LAE &/or LVH) documented on echo
HF hospitalization within past 12 months
Key exclusions:
SBP <100 mm Hg
eGFR <20
BMI ≥45
SGLT2i contraindication (history of ketoacidosis, allergy/hypersensitivity)
“Cardiovascular (CV) disease/treatment that increase the unpredictability of or change the patients’ clinical course independent of HF” (e.g. MI/stroke/TIA/CV surgery in past 90 days; infiltrative cardiomyopathy; heart transplant recipient/wait list; severe valvular disease)
“Untreated CV condition that might influence the course of HF/study drug tolerability” (e.g. AF with uncontrolled HR, SBP ≥180 mm Hg)
“Significant comorbidity that might influence clinical course” (e.g. pulmonary disease requiring O2, PO steroids or requiring hospitalization; acute/chronic liver disease)
Baseline characteristics:
Age 72, 45% female, 76% White/14% Asian
NYHA 2 (81%), 3 (18%)
Mean LVEF 54% (~1/3 each in categories 41-49%, 50-59%, ≥60%)
Median NT-proBNP ~950-1000 pg/mL
HF hospitalization in last 12 months ~23%
Comorbidities: HTN 90-91%, AF 51%, eGFR <60 50%, diabetes 49%
Meds: Beta-blocker 86%, ACEI/ARB 79%, ARNI ~2%, MRA 37-38%, digitalis 9-10%
SBP 132, HR 70
Intervention: Empagliflozin 10 mg qAM
Comparator: Matching placebo
Outcomes at median 26.2 months (2.2 years)
Effect on quality of life (using Kansas City Cardiomyopathy Questionnaire [KCCQ]; range 0 [worst] to 100 [best]):
More likely to have a clinically-important (≥5/100) improvement in quality of life with empagliflozin vs placebo
KCCQ-overall summary score at 1 year: Empagliflozin 49.6% vs placebo 45.8% (+3.8%)
Similar effect over time (e.g. difference +4.7% at 3 months vs 3.8% at 12 months)
Similar difference if considering clinically-important decline (-4.8% at 1 year) or different cutoffs for improvement (+2.3% for ≥10-point improvement & +3.6% for ≥15-point improvement)
Similar difference if considering KCCQ subscores (e.g. +4.6% for KCCQ-total symptoms score [HF symptom burden + frequency] at 1 year)
Cumulative incidence curve for the primary composite outcome showing immediate separation of empagliflozin and placebo curves (suggesting early benefit)
Effect on biometrics & biomarkers (difference vs placebo):
Body weight: -1.3 kg
SBP -1.2 mm Hg
A1c: -0.2%
NT-proBNP: -20 pg/mL
Internal validity: Low risk of bias
Computer-generated random sequence using permuted blocks
Stratified by geographic region, diabetes status, eGFR <60 or ≥60, & LVEF <50% or ≥50%
Allocation concealment by central randomization via interactive response technology
Blinding of participants and treating clinicians with matching placebo
Blinded outcome adjudication
Intention-to-treat analysis
3% loss-to-follow-up for primary outcome, 0.6% for death
Other considerations
Are the results clinically important?
Maybe; this will very much depend on individual patient/clinician preferences
Overall, likely net clinical benefit based on composite of % who died or had a hospitalization due to any cause
HF hospitalizations only accounted for 18% of total hospitalization outcomes in this trial, and therefore the 3.2% absolute reduction in the risk of a first HF hospitalization is diluted in total hospitalizations
Neutral effect on all-cause death & inconclusive effect on CV deaths
CV death accounted for 55% of deaths (sudden death > HF > other), & non-CV deaths accounted for 45% (infection > malignancy > other)
QoL improvement with empagliflozin consistent with results of the PRESERVED-HF trial & effects of SGLT2i on QoL in HFrEF trials
Brief summary of PRESERVED-HF:
P: 324 patients with NYHA 2-4 HF & LVEF >=45% (mixed HFpEF/HFmrEF) + elevated NT-proBNP/BNP + receiving a diuretic + additional enrichment criteria + eGFR >=20 + SBP >=100
I: Dapagliflozin 10 mg daily
C: Placebo
O: KCCQ-23 @ 3 months
Mean +4.5/100 in overall-summary score with dapa
Clinically-important improvement: Dapa 45.4% vs placebo 34.9% (+10.5%) at 3 months
How do we apply these results to patient care (generalizability)?
Although the study defined “preserved” ejection fraction as >40%, the 2021 universal definition and classification of HF further sub-classify HF as HFmrEF if 41-49% (~1/3 of the study population) & HFpEF if ≥50%
Subgroup analysis of the primary outcome comparison based on baseline LVEF suggested attenuation of efficacy with increasing LVEF, with uncertain efficacy with LVEF ≥60%
Hazard ratio progressively attenuated from LVEF 41-49% (0.71, 95% CI 0.57-0.88), 50-59% (0.80, 95% CI 0.64-0.99), ≥60% (0.87, 0.69-1.10)
Risk of the primary outcome increased with lower LVEF, leading to a greater absolute risk reduction in those with lower baseline LVEF (even if we assume constant 21% relative risk reduction regardless of LVEF)
LVEF 41-49%: Risk in placebo group 19.5%, absolute risk reduction 4.1%
LVEF 50-59%: Risk in placebo group 16.8%, absolute risk reduction 3.5%
LVEF ≥60%: Risk in placebo group 14.9%, absolute risk reduction 3.1%
Efficacy on primary outcome (in terms of relative effect) similar in females/males, diabetes/no diabetes, AF/no AF, eGFR <60/≥60, & regardless of race/ethnicity
More to come…